All everyday objects that can be touched are ultimately composed of atoms which are made up of interacting subatomic particles and in everyday as well as scientific usage matter generally includes atoms and anything made up. For example we should not write a structure in which carbon has five bonds.

Resonance Structures Basic Introduction How To Draw The Resonance Hybrid Chemistry Youtube
In drawing resonance structures for a molecule we are only allowed to move electrons.
. Write the CSS and draw the resonance hybrid of each of the following. To find the resonance structure of ozone we will draw the lewis structure of ozone. Some familiarity with the structure of organic functional groups would be.
The atoms must have the same position. View solution View. The net charge on the central atom remains 1.
Resonance structures of the nitrate ion The nitrate ion has three valid contributing structures that vary according to the placement of the electrons. Draw the resonance structures for the following compound. Start your trial now.
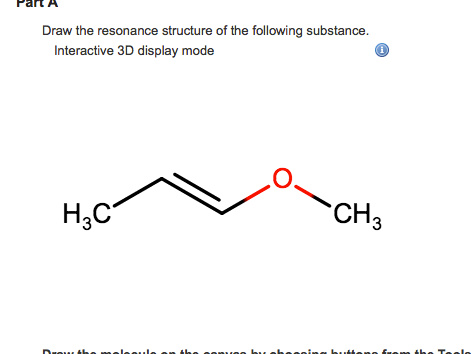
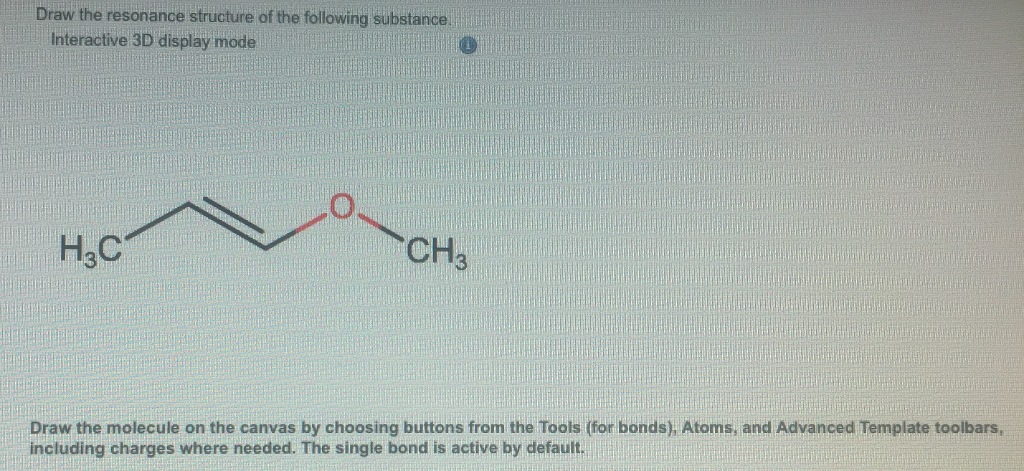
It gives the following structure and has a multiple bonds and an adjacent atom with one lone pair of electrons. Interactive 3D display mode HC CH3 Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars including charges where needed. We can obtain the following resonance structures by following the same steps as mentioned above.
This resonance hybrid is illustrated below. Draw the resonance structure of the following substance. Click hereto get an answer to your question Draw the resonance structures of the following compounds.
Since the molecular formula is O 3 we know there are 18 valence electrons oxygen has six valence electrons as 6 x 3 18. By signing up youll get thousands of step-by-step solutions to your homework. Interactive 3D display mode.
In resonance structures it does not require to show transformation of electrons by arrows. And this means you should never place more than eight electrons on those ie. In this article I will assume you are familiar with the idea of equilibrium know how to deduce the formal charge of atoms in molecules and polyatomic ions and lastly know that double bonds are shorter than single bonds.
When you are drawing resonance structures it is important to remember to shift only the electrons. Draw the Lewis structure for CO32- including any valid resonance structures. Interactive 3D display mode.
If a resonance hybrid of this polyatomic ion is drawn from the set of Lewis structures provided above the partial charge on each oxygen atom will be equal to - ⅔. After placing all the electrons we will have a double bond and a single bond. None of the above are true.
The three possible resonance structures of NO 3 are illustrated below. Subtract this number from the total number of valence electrons in benzene and then locate the remaining electrons such that each atom in the structure reaches an octet. View solution Find the substance which can form a resonance structure.
There are many types of the carbocation is formed in different chemical reactions. The curved arrow in structure B represents type 2 resonance motion - the pi bond breaks to form a new pi bond to the carbocation carbon. The oxygens share the negative charge with each other stabilizing it and reducing the charge on either atom.
The CO32- ion contains three CO double bonds. Draw the resonance structure of the following substance. I CH2 CH - Cl.
In following examples arrows are used to show electrons transformation. Draw a structure for benzene illustrating the bonded atoms. I CH2 CH - Cl.
Then calculate the number of valence electrons used in this drawing. Ii CH2 CH - CH CH2 iii CH2 CH - H C O. Draw the resonance structures for benzene.
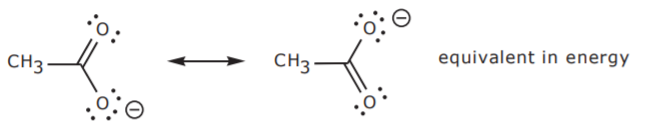
Resonance Structures are a representation of a Resonance Hybrid which is the combination of all resonance structures. Interactive 3D display mode. This is important because neither resonance structure actually exists instead there is a hybrid.
Draw the resonance structure of the following substance. Lewis structures and resonance. View solution Resonance structures differ only in the arrangement of _____.
Solution for Draw the condensed or the line structure for the following compounds and give their name i an aldehyde functional containing 6 carbons ii an. The resonance structure with the Formal Charge closest to zero is the most accepted structure however the correct Lewis structure is actually a combination of all the resonance structures and is not solely describe as one. Drawing correct resonance structures.
Draw the resonance structures for the following compound. Ii CH2 CH - CH CH2 iii CH2 CH - H C O Solve Study Textbooks Guides. First week only 499.
The second-row elements C N O F can only handle up to eight electrons because of their orbitals. The curved arrow in structure A represents the type 3 resonance motion - the pi bond between the carbon and oxygen breaks to form another lone pair on the oxygen. Up to 256 cash back Get the detailed answer.
Draw the Lewis Structure Resonance. To draw all resonance structures take the lewis structure we drawn by using VESPR rule. Classification and Properties of Matter.
Draw the resonance structure of the following substance. Oc 0 H 120 ex cont с N O HC сн. In chemistry and physics matter is any substance that has mass and takes up space by having volume.
Drawing the Lewis Structure of Ozone. You must follow the octet rule. All of the resonance structures must be proper Lewis structures.
Draw the resonance structure of the following substance. Weve got the study and writing resources you need for your assignments. Experts are tested by Chegg as specialists in their subject area.
The following geometries may be used. The CO32- ion contains two CO single bonds and one CO double bond. The CO32- ion contains one CO single bond and two CO double bonds.
Therefore we can draw resonance structures for O 3 molecule as follows. Draw the resonance structure of the following substance It is simpler than uncomplicated to create superb nail artwork for short nailsAll you need to do will be to introduce some glitter in. While both resonance structures are chemically identical the negative charge is on a different oxygen in each.
The single bond is active by default. But to identify each resonance structures it is good to show arrows. 1 Do not exceed the octet on 2nd-row elements.
Draw the resonance structure of the following substance. The positions of all nuclei must remain the same. Which of the following statements is TRUE.
We review their content and use your feedback to keep the quality high. View solution In the following the least stable resonance structure is. Rules for drawing resonance structures.
Draw the resonance structure of the following substance.
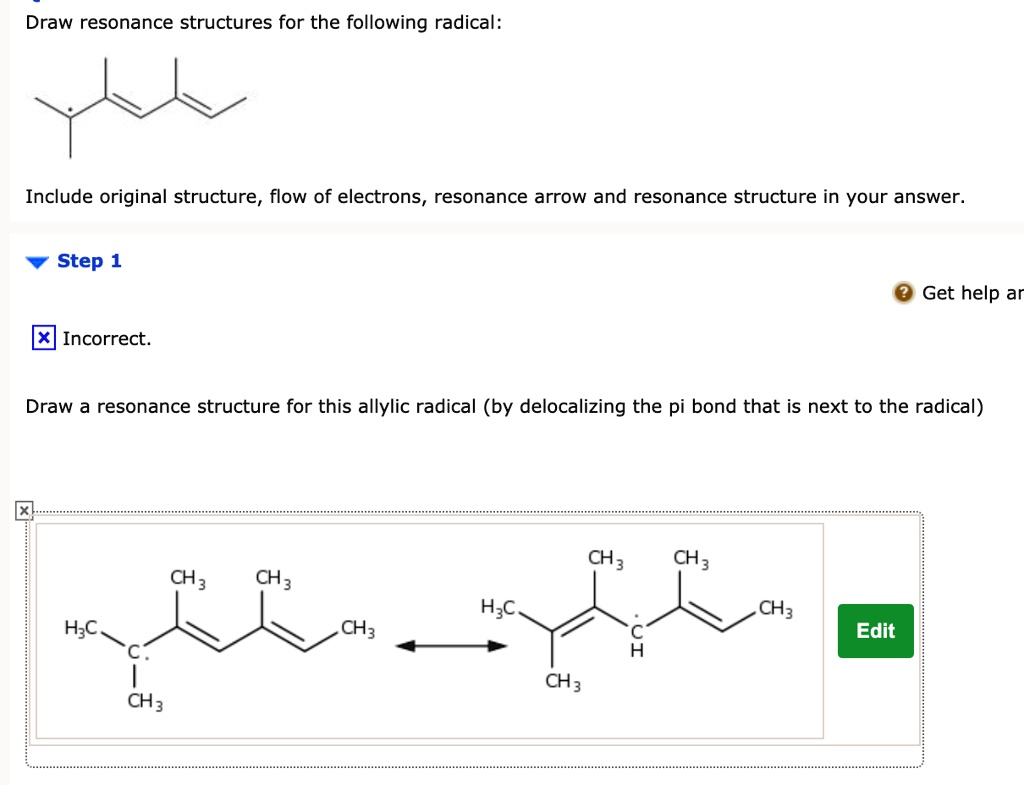
Solved Draw Resonance Structures For The Following Radical Include Original Structure Flow Of Electrons Resonance Arrow And Resonance Structure In Your Answer Step 1 Get Help Ar Incorrect Draw A Resonance Structure For

Resonance Structures And Calculated Mulliken Charges Of No 3 A Download Scientific Diagram
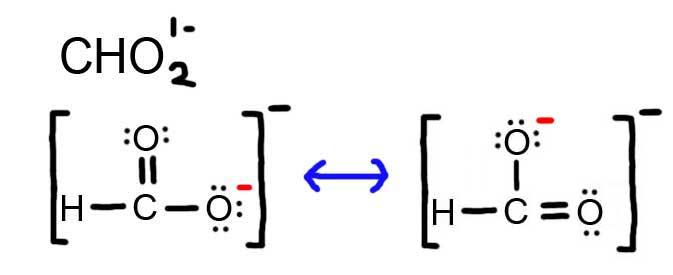
7 4 How To Draw Resonance Contributors Chemistry Libretexts

Solved Draw The Resonance Structure Of The Following Chegg Com
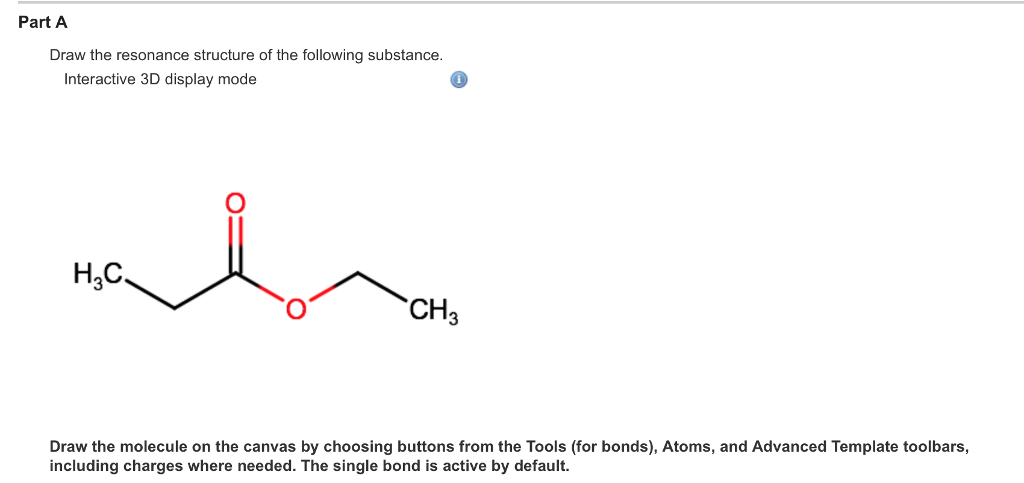
Solved Draw The Resonance Structure Of The Following Chegg Com
Solved Draw The Resonance Structure Of The Following Chegg Com
0 comments
Post a Comment